
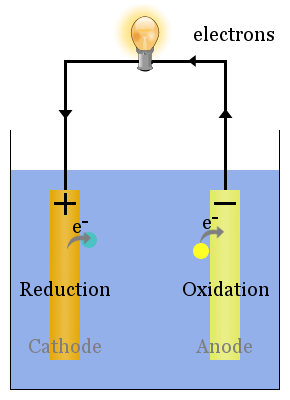
(the “Gold Book”) Blackwell Scientific Publications: Oxford, 1997. The anode and cathode must both be in this environment enabling cathodic protection current to flow from the anode to the cathode. International Union of Pure and Applied Chemistry, IUPAC Compendium of Chemical Terminology, 2nd ed. Notes and Records of the Royal Society of London 1961, 16, 187–220. The cathode is also called the positive terminal. Thus, the cathode is the electrode where reduction occurs. The word cathode comes from the Greek κάθοδος ( kathodos), meaning ‘descent’ or ‘the way downward’, which today refers to the movement of electrons down from the circuit into the electrode where they facilitate electrochemical reduction of the positive ions in the electrolyte solution at the surface of the cathode. The anode is also called the negative terminal. Thus, the anode is the electrode where oxidation occurs. Meanwhile, in a traditional cell, the positive ions resulting from the same oxidation enter into the electrolyte solution. The word anode comes from the Greek ἄνοδος ( anodos), meaning ‘ascent’ or ‘the way upward’, which today refers to the movement of electrons up into the circuit after being released from the electrode material by oxidation. In the course of my own study of electrochemistry, I thought other students may find this information helpful in keeping everything straight. The following is a brief summary of their etymology and their meaning as it stands today in electrochemical circuits. As scientists have learned about how electrochemistry works, the definitions have evolved somewhat. A delightful (and highly recommended) historical account of how these words were conceived by Faraday and his associates can be found in Faraday Consults the Scholars: The Origins of the Terms of Electrochemistry by Sydney Ross. This requires only two pins from the Arduino (data & clock), and they can be chained to have multiple 7-segments driven from those same 2 pins.The terms “anode” and “cathode” were first published by Michael Faraday, F.R.S. This limits you to being able to display only numerical digits, but uses only 4 pins of the Arduino per 7-segment.Īnother, more versatile way is to add a serial-in to parallel out chip (74HC595, for example) to drive the 7-segment. One is to add a 7-segment driver, like the CD4511. There are a couple of ways to address this. You will run out of digital pins if you try to add a second 7-segment.

The way that you are diving the 7-segment involves using a separate pin to drive each segment, so you use 8 pins of the Arduino to drive the display. So driving one of these means running a current from the particular anode (positive) pin for the desired segment to the common cathode pin.
INTRODUCTION Lithium-ion batteries are used in different technologies such as the Hybrid Electric Vehicles (HEV), which use both battery as well as electric motor engines to increase the fuel efficiency 1. Key Differences Between Anode and Cathode The key factor of differentiation between anode and cathode is that anode corresponds to the electrode where oxidation i.e., loss of electrons occurs. So turning on any particular segment will involve running a current from this common anode (positive) pin to the particular cathode (negative) pin for the desired segment.Ĭommon cathode means that the cathodes of all of the LEDs are common and connected to a single pin. Index TermsCathode, Anode, Graphite, Lithium ion, Battery, Safety I. Common anode means that the anode (positive) side of all of the LEDs are electrically connected at one pin, and each LED cathode has its own pin. At the anode, anions (negative ions) are. PinMode(LED8, OUTPUT) // led 8 is outputĪ 7-segment is a packaged set of 8 LEDs (7 number-segments & 1 decimal point). In electrochemistry, the anode is where oxidation occurs and is the positive polarity contact in an electrolytic cell. PinMode(LED1, OUTPUT) // led are output for low will be on Therefore, the anode has a negative charge. PinMode(ANODE, OUTPUT) // common anode is obviously an output The oxidized species lose electrons by leaving electrode with an accumulation of electrons.
Cathode vs anode code#
How can you change the code to have a counter in backwards (from 0 to 9)? const int ANODE = 2 What is the difference in using a common anode and common cathode of the 7 Segment in interfacing with the Arduino? How can you change the code if we need to add another 7 Segment display?


 0 kommentar(er)
0 kommentar(er)
